
Chemistry, 18.08.2020 20:01 epmooneyham922
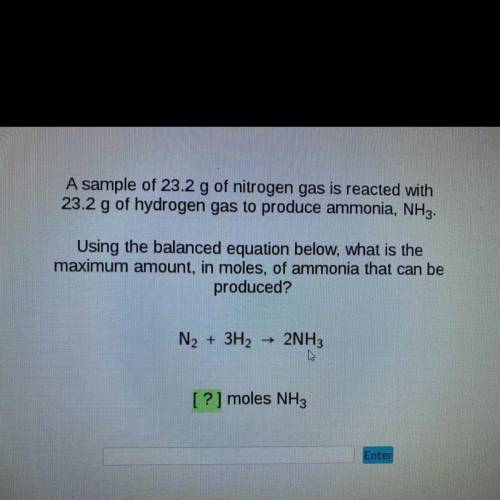
A sample of 23.2 g of nitrogen gas is reacted with
23.2 g of hydrogen gas to produce ammonia, NH3.
Using the balanced equation below, what is the
maximum amount, in moles, of ammonia that can be
produced?
N2 + 3H2
2NH3
IN
[?] moles NH3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1
You know the right answer?
A sample of 23.2 g of nitrogen gas is reacted with
23.2 g of hydrogen gas to produce ammonia, NH3.<...
Questions

Mathematics, 09.05.2021 21:30


History, 09.05.2021 21:30

Chemistry, 09.05.2021 21:30


Mathematics, 09.05.2021 21:30

Chemistry, 09.05.2021 21:30

Mathematics, 09.05.2021 21:30

Mathematics, 09.05.2021 21:30

Chemistry, 09.05.2021 21:30


Mathematics, 09.05.2021 21:30


History, 09.05.2021 21:30

Mathematics, 09.05.2021 21:30

Computers and Technology, 09.05.2021 21:30


English, 09.05.2021 21:30

Mathematics, 09.05.2021 21:30



