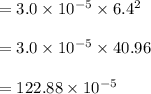Chemistry, 17.08.2020 01:01 nicholasferrell
The reaction NO2(g) + CO(g) → NO(g) + CO2(g) has been found to be second order with respect to NO2 and zero order with respect to CO. At a certain temperature, the rate constant is found experimentally to be 3.0 × 10−5 L mol · s . What is the rate of formation

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2
You know the right answer?
The reaction NO2(g) + CO(g) → NO(g) + CO2(g) has been found to be second order with respect to NO2 a...
Questions


Mathematics, 15.07.2019 11:30

Mathematics, 15.07.2019 11:30




Advanced Placement (AP), 15.07.2019 11:30




Mathematics, 15.07.2019 11:30

Mathematics, 15.07.2019 11:30




Social Studies, 15.07.2019 11:30

Mathematics, 15.07.2019 11:30



English, 15.07.2019 11:30

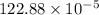 "
"

![=k.[NO_2]^2](/tpl/images/0723/2177/ff6c8.png) because the above given is the part of the second-order, which relates to
because the above given is the part of the second-order, which relates to 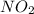 . In the zeros order the Carbon monoxide (CO) its reaction doesn't affect the rate.
. In the zeros order the Carbon monoxide (CO) its reaction doesn't affect the rate.