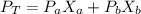Chemistry, 16.08.2020 14:01 ant5784tgi
Two volatile substances, A and B are dissolved in one another and the resulting solution has a vapor pressure of 137 torr. If the mole fraction of B is 0.230 and the vapor pressure of pure B is 162 torr, what is the vapor pressure of pure A in torr?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

You know the right answer?
Two volatile substances, A and B are dissolved in one another and the resulting solution has a vapor...
Questions

Mathematics, 31.03.2021 19:20





Physics, 31.03.2021 19:20

Biology, 31.03.2021 19:20

Mathematics, 31.03.2021 19:20



Chemistry, 31.03.2021 19:20

Mathematics, 31.03.2021 19:20


Mathematics, 31.03.2021 19:20

History, 31.03.2021 19:20

English, 31.03.2021 19:20

Mathematics, 31.03.2021 19:20

Chemistry, 31.03.2021 19:20

History, 31.03.2021 19:20

History, 31.03.2021 19:20