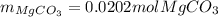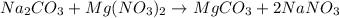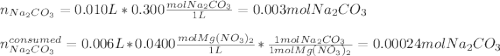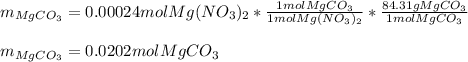Chemistry, 15.08.2020 20:01 leeenaaa95
Calculate the mass of MgCO3 (84.31 g/mol) precipitated by mixing 10.0 mL of a 0.300 M Na2CO3 solution with 6.00 mL of 0.0400 M Mg(NO3)2 solution.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1


Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2
You know the right answer?
Calculate the mass of MgCO3 (84.31 g/mol) precipitated by mixing 10.0 mL of a 0.300 M Na2CO3 solutio...
Questions

Mathematics, 27.04.2021 21:10

Chemistry, 27.04.2021 21:10

Mathematics, 27.04.2021 21:10

Chemistry, 27.04.2021 21:10



Social Studies, 27.04.2021 21:10




Mathematics, 27.04.2021 21:10


History, 27.04.2021 21:10


History, 27.04.2021 21:10

Mathematics, 27.04.2021 21:10


History, 27.04.2021 21:10

Mathematics, 27.04.2021 21:10

Mathematics, 27.04.2021 21:10