
Chemistry, 15.08.2020 08:01 kingofmortals2119
Why is calcium chloride (rock salt, CaCl2) more effective than sodium chloride (table salt, NaCl) at de-icing roads in the winter? A. Calcium chloride only breaks into 2 ions, while sodium chloride does not break up and remains 1 ion. B. Calcium is a larger molecule than sodium, so it has a greater effect. C. The additional chlorine atom forms a stronger bond between the atoms in calcium chloride than in sodium chloride, so calcium chloride remains one molecule while sodium chloride breaks apart. D. Calcium chloride breaks into 3 ions, while sodium chloride only breaks into 2 ions.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3
You know the right answer?
Why is calcium chloride (rock salt, CaCl2) more effective than sodium chloride (table salt, NaCl) at...
Questions


Mathematics, 07.03.2020 03:39

Mathematics, 07.03.2020 03:39



Computers and Technology, 07.03.2020 03:39







Mathematics, 07.03.2020 03:40



Mathematics, 07.03.2020 03:40




Mathematics, 07.03.2020 03:40

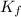 × m × i
× m × i

