
Chemistry, 13.08.2020 18:01 sampurple123
Dinitrogen tetroxide and hydrazine (N2H4) undergo a redox reaction in which nitrogen and water are formed as products. What mass of nitrogen could be produced when 50.0 g of dinitrogen tetroxide and 45.0 g of hydrazine are combined?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
Dinitrogen tetroxide and hydrazine (N2H4) undergo a redox reaction in which nitrogen and water are f...
Questions


Mathematics, 07.12.2020 20:00


Mathematics, 07.12.2020 20:00

Mathematics, 07.12.2020 20:00


History, 07.12.2020 20:00



Mathematics, 07.12.2020 20:00




Arts, 07.12.2020 20:00

Mathematics, 07.12.2020 20:00




Mathematics, 07.12.2020 20:00

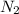 = 45.65 g
= 45.65 g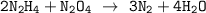
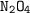 :
: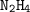 :
:

