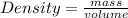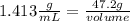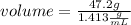Chemistry, 14.08.2020 01:01 reginaldboyd28
The density of concentrated nitric acid (HNO3) is 1.413 g/mL. What volume in liters would be occupied by a mass of 47.2 g?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1


Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
The density of concentrated nitric acid (HNO3) is 1.413 g/mL. What volume in liters would be occupie...
Questions


English, 20.09.2019 21:20













Computers and Technology, 20.09.2019 21:20