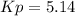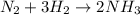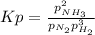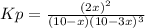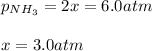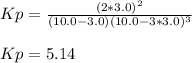Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
a reaction mixture initially contains 10.0 atm N2 and 10.0 atm H2. If the equilibrium pressure of NH...
Questions


Geography, 20.01.2020 10:31


Mathematics, 20.01.2020 10:31



Mathematics, 20.01.2020 10:31






English, 20.01.2020 10:31

Mathematics, 20.01.2020 10:31

Chemistry, 20.01.2020 10:31



History, 20.01.2020 10:31