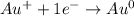Chemistry, 13.08.2020 04:01 dbhuggybearow6jng
PLEASE HELP!!
You are performing an experiment that involves the electrolysis of gold (I) bromide, also know as AuBr. You know that gold is less reactive than hydrogen. Which of the following would be the product of the reaction?
A. Hydrogen gas
B. Gold bromide
C. Oxygen gas
D. Pure gold

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2

You know the right answer?
PLEASE HELP!!
You are performing an experiment that involves the electrolysis of gold (I) bromide,...
Questions



Mathematics, 14.05.2021 22:50

History, 14.05.2021 22:50

Arts, 14.05.2021 22:50


Mathematics, 14.05.2021 22:50


Mathematics, 14.05.2021 22:50


Mathematics, 14.05.2021 22:50

English, 14.05.2021 22:50



Spanish, 14.05.2021 22:50

Mathematics, 14.05.2021 22:50


Mathematics, 14.05.2021 22:50


Physics, 14.05.2021 22:50