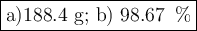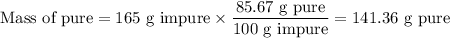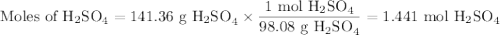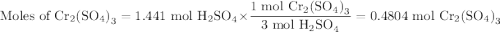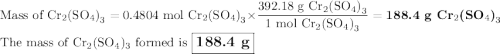Chromium is dissolved in sulfuric acid according to the following equation: Cr + H2SO4 ⇒ Cr2 (SO4) 3 + H2
a) How many grams of Cr2 (SO4) 3 can be obtained by reacting 165 g of 85.67% H2SO4 of purity?
b) If 485.9 g of Cr2 (SO4) 3 are obtained, what is the yield of the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3


Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
Chromium is dissolved in sulfuric acid according to the following equation: Cr + H2SO4 ⇒ Cr2 (SO4) 3...
Questions

Mathematics, 27.09.2019 12:30






Mathematics, 27.09.2019 12:30


Chemistry, 27.09.2019 12:30

Mathematics, 27.09.2019 12:30



Biology, 27.09.2019 12:30



Social Studies, 27.09.2019 12:30




Mathematics, 27.09.2019 12:30