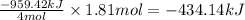Chemistry, 12.08.2020 04:01 21121212cutecheytown
4NH3(g) 5O2(g)4NO(g) 6H2O(g) Using standard thermodynamic data at 298K, calculate the free energy change when 1.81 moles of NH3(g) react at standard conditions.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3
You know the right answer?
4NH3(g) 5O2(g)4NO(g) 6H2O(g) Using standard thermodynamic data at 298K, calculate the free energy ch...
Questions



Mathematics, 15.04.2021 20:50

Mathematics, 15.04.2021 20:50

Mathematics, 15.04.2021 20:50





Biology, 15.04.2021 20:50


Social Studies, 15.04.2021 20:50



Mathematics, 15.04.2021 20:50


History, 15.04.2021 20:50

History, 15.04.2021 20:50