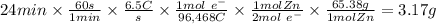Chemistry, 12.08.2020 06:01 sparkybig12
Zinc is used as a coating for steel to protect the steel from environmental corrosion. If a piece of steel is submerged in an electrolysis bath for 24 minutes with a current of 6.5 Amps, how many grams of zinc will be plated out? The molecular weight of Zn is 65.38, and Zn+2 + 2e– → Zn. Question 7 options: A) 3.17 g of Zn B) 1.09 g of Zn C) 6.34 g of Zn D) 12.68 g of Zn

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
You know the right answer?
Zinc is used as a coating for steel to protect the steel from environmental corrosion. If a piece of...
Questions



Mathematics, 03.02.2020 04:05

Mathematics, 03.02.2020 04:05


Biology, 03.02.2020 04:05

History, 03.02.2020 04:05


Mathematics, 03.02.2020 04:05



Spanish, 03.02.2020 04:05

Mathematics, 03.02.2020 04:05

Chemistry, 03.02.2020 04:05

Biology, 03.02.2020 04:05

Chemistry, 03.02.2020 04:05


History, 03.02.2020 04:05

Chemistry, 03.02.2020 04:05

History, 03.02.2020 04:05