
Chemistry, 12.08.2020 06:01 kayleg907436
In the buffer solution image Question 1 options: A) CH3CO2H is a base, and H3O+ is its conjugate acid. B) H3O+ is an acid, and CH3CO2 – is its conjugate base. C) CH3CO2H is an acid, and CH3CO2 – is its conjugate base. D) H3O+ is an acid, and CH3CO2H is its conjugate base.
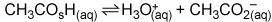

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 14:00
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature.
Answers: 1

Chemistry, 23.06.2019 18:20
Consider the following system at equilibrium. caco3(s) = ca2+(aq) + co32-(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? occia co2 cuso4 na2co3
Answers: 3
You know the right answer?
In the buffer solution image Question 1 options: A) CH3CO2H is a base, and H3O+ is its conjugate aci...
Questions


Mathematics, 07.10.2019 14:00


History, 07.10.2019 14:00








Physics, 07.10.2019 14:00



Biology, 07.10.2019 14:00

Mathematics, 07.10.2019 14:00







