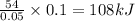Chemistry, 12.08.2020 05:01 helloyall40
A student mixed 50 ml of 1.0 M HCl and 50 ml of 1.0 M NaOH in a coffee cup calorimeter and calculate the molar enthalpy change of the acid-base neutralization reaction to be –54 kJ/mol. He next tried the same experiment with 100 ml of 1.0 M HCl and 100 ml of 1.0 M NaOH. The calculated molar enthalpy change of reaction for his second trial was:

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2
You know the right answer?
A student mixed 50 ml of 1.0 M HCl and 50 ml of 1.0 M NaOH in a coffee cup calorimeter and calculate...
Questions

Chemistry, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

Medicine, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01

Computers and Technology, 16.10.2020 18:01


Medicine, 16.10.2020 18:01

History, 16.10.2020 18:01

Mathematics, 16.10.2020 18:01


Mathematics, 16.10.2020 18:01




Mathematics, 16.10.2020 18:01

Geography, 16.10.2020 18:01


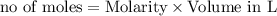
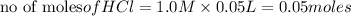
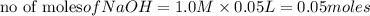
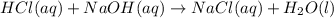
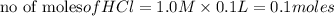
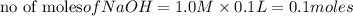
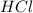 reacts with 0.05 moles of
reacts with 0.05 moles of 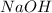 to release heat = 54 kJ
to release heat = 54 kJ