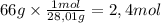Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Calcular el volumen que ocupa 66 gramos de CO medido a 27°C y 624 mm Hg Dato: Masas atómicas: C= 12...
Questions

History, 30.03.2020 16:28


Biology, 30.03.2020 16:29


Business, 30.03.2020 16:29











Mathematics, 30.03.2020 16:32


Mathematics, 30.03.2020 16:33


Biology, 30.03.2020 16:33