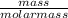Chemistry, 12.08.2020 06:01 genyjoannerubiera
g What is the molarity of hydrochloric acid if 40.95 mL of HCl is required to neutralize 0.550 g of sodium oxalate, Na2C2O4

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

You know the right answer?
g What is the molarity of hydrochloric acid if 40.95 mL of HCl is required to neutralize 0.550 g of...
Questions

English, 01.07.2019 21:20


English, 01.07.2019 21:20

Mathematics, 01.07.2019 21:20
















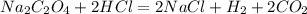
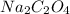 reacts with 2 moles of
reacts with 2 moles of 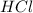 for a complete neutralization reaction.
for a complete neutralization reaction.