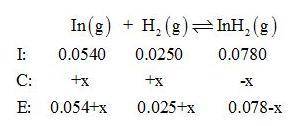Chemistry, 05.08.2020 16:01 XxDonaldTrumpxX452
Gaseous indium dihydride is formed from the elements at elevated temperature:
In(g)+H2(g)⇌InH2(g),Kp=1.48 at 973 K
The partial pressures measured in a reaction vessel are
PIn =0.0540atm
PH2= 0.0250atm
PInH2 =0.0780atm
Calculate Qp and give equal partial pressure for In, H2, and InH2.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1
You know the right answer?
Gaseous indium dihydride is formed from the elements at elevated temperature:
In(g)+H2(g)⇌InH2(g),...
Questions



Mathematics, 04.12.2020 19:40


Chemistry, 04.12.2020 19:40

Mathematics, 04.12.2020 19:40




Mathematics, 04.12.2020 19:40

English, 04.12.2020 19:40

Mathematics, 04.12.2020 19:40

Mathematics, 04.12.2020 19:40

Mathematics, 04.12.2020 19:40

Arts, 04.12.2020 19:40



Mathematics, 04.12.2020 19:40

Physics, 04.12.2020 19:40

History, 04.12.2020 19:40