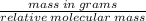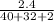Chemistry, 04.08.2020 01:01 smelcher3900
There are 2.4g of calcium hydroxide reacted with nitric acid. Calculate the number of moles of calcium hydroxide used. Write your answer using proper significant digits and units. Show all your work.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
There are 2.4g of calcium hydroxide reacted with nitric acid. Calculate the number of moles of calci...
Questions

Geography, 07.03.2021 08:30

Mathematics, 07.03.2021 08:30

Mathematics, 07.03.2021 08:30



History, 07.03.2021 08:30

English, 07.03.2021 08:30

English, 07.03.2021 08:30




English, 07.03.2021 08:30

History, 07.03.2021 08:30






Mathematics, 07.03.2021 08:40