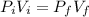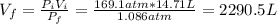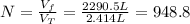A cylinder containing 14.71 L of helium gas at a pressure of 169.1 atm is to be used to fill toy balloons to a pressure of 1.086 atm. Each inflated balloon has a volume of 2.414 L. What is the maximum number of balloons that can be inflated? Report your answer to 1 decimal place. (Remember that 14.71 L of helium at 1.086 atm will remain in the exhausted (empty) cylinder)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1



Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
A cylinder containing 14.71 L of helium gas at a pressure of 169.1 atm is to be used to fill toy bal...
Questions



Mathematics, 07.11.2019 03:31

Geography, 07.11.2019 03:31

Biology, 07.11.2019 03:31

Mathematics, 07.11.2019 03:31


History, 07.11.2019 03:31











Biology, 07.11.2019 03:31

Biology, 07.11.2019 03:31

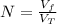
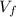 : is the volume at 1.086 atm
: is the volume at 1.086 atm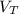 : is the balloon volume = 2.414 L
: is the balloon volume = 2.414 L