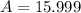In nature, oxygen has three common isotopes. The atomic masses and relative abundances of these isotopes are given in the table below. Isotope Atomic Mass (amu) Relative Abundance O-16 15.995 99.759% O-17 16.995 0.037% O-18 17.999 0.204% Calculate the average atomic mass of oxygen. Show all of your calculations below.

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
You know the right answer?
In nature, oxygen has three common isotopes. The atomic masses and relative abundances of these isot...
Questions

English, 19.11.2020 14:00


Arts, 19.11.2020 14:00

Mathematics, 19.11.2020 14:00


Chemistry, 19.11.2020 14:00


English, 19.11.2020 14:00

Mathematics, 19.11.2020 14:00

Arts, 19.11.2020 14:00

Mathematics, 19.11.2020 14:00

Computers and Technology, 19.11.2020 14:00

English, 19.11.2020 14:00

Chemistry, 19.11.2020 14:00

Mathematics, 19.11.2020 14:00


Arts, 19.11.2020 14:00


Arts, 19.11.2020 14:00

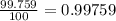
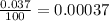
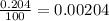
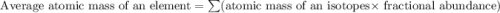
![A=\sum[(15.995\times 0.99759)+(16.995\times 0.00037)+(17.999 \times 0.00204)]](/tpl/images/0715/9581/687c5.png)