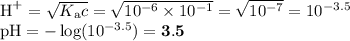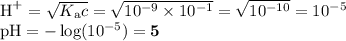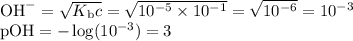Chemistry, 31.07.2020 01:01 seasmarie75
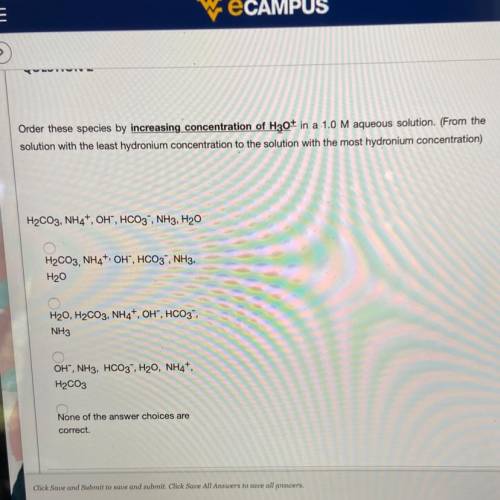
Order these species by increasing concentration of H30+ in a 1.0 M aqueous solution. (From the
solution with the least hydronium concentration to the solution with the most hydronium concentration)
NO
H2CO3, NH4, OH, HCO3, NH3, H20
Home
ir
H2CO3,NH4+, OH", HCO3, NH3,
H20
Paste
H20, H2CO3, NH4+, OH", HCO3-
NH3
6
con
O
OH", NH3, HCO3, H20, NH4+,
H2CO3
None of the answer choices are
correct.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1



Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
You know the right answer?
Order these species by increasing concentration of H30+ in a 1.0 M aqueous solution. (From the
solu...
Questions

Mathematics, 05.05.2021 01:00



English, 05.05.2021 01:00

Chemistry, 05.05.2021 01:00

Mathematics, 05.05.2021 01:00



Mathematics, 05.05.2021 01:00

Mathematics, 05.05.2021 01:00

Mathematics, 05.05.2021 01:00



English, 05.05.2021 01:00



Mathematics, 05.05.2021 01:00

Mathematics, 05.05.2021 01:00


Mathematics, 05.05.2021 01:00