
Chemistry, 30.07.2020 23:01 JGottem6489
A certain reaction has the following general form. aA → bB At a particular temperature and [A]0 = 2.80 ✕ 10−3 M, concentration versus time data were collected for this reaction, and a plot of 1/[A] versus time resulted in a straight line with a slope value of +3.40 ✕ 10−2 L mol−1 s−1. (a) Determine the rate law, the integrated rate law, and the value of the rate constant for this reaction. (Rate expressions take the general form: rate = k . [A]a . [B]b.) rate law:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

You know the right answer?
A certain reaction has the following general form. aA → bB At a particular temperature and [A]0 = 2....
Questions



Spanish, 30.10.2020 18:50

Mathematics, 30.10.2020 18:50

Mathematics, 30.10.2020 18:50



Mathematics, 30.10.2020 18:50


Arts, 30.10.2020 18:50

Chemistry, 30.10.2020 18:50


Mathematics, 30.10.2020 18:50

History, 30.10.2020 18:50


Mathematics, 30.10.2020 18:50

Mathematics, 30.10.2020 18:50

Spanish, 30.10.2020 18:50


![r=k[A]^2](/tpl/images/0715/7128/d4e0e.png)
![\frac{1}{[A]}=kt+ \frac{1}{[A]_0}](/tpl/images/0715/7128/acbfc.png)
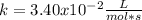
![\frac{d[A]}{dt}=-k[A]^2](/tpl/images/0715/7128/47b67.png)



