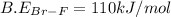Chemistry, 29.07.2020 16:01 brookeboyd7469
Consider the following reaction: Br2(g) + 3 F2(g) LaTeX: \rightarrow→ 2 BrF3(g) LaTeX: \Delta H_{rxn}Δ H r x n= ‒836 kJ/mol Bond Bond Energy (kJ/mol) Br–Br 193 F–F 155 Using the above bond dissociation energies, calculate the energy, in kJ/mol, of a Br–F bond.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
Consider the following reaction: Br2(g) + 3 F2(g) LaTeX: \rightarrow→ 2 BrF3(g) LaTeX: \Delta H_{rxn...
Questions


Mathematics, 24.06.2021 01:30




Mathematics, 24.06.2021 01:30

Mathematics, 24.06.2021 01:30


English, 24.06.2021 01:30


English, 24.06.2021 01:30


Mathematics, 24.06.2021 01:40

Mathematics, 24.06.2021 01:40

Mathematics, 24.06.2021 01:40

Mathematics, 24.06.2021 01:40

Chemistry, 24.06.2021 01:40


Mathematics, 24.06.2021 01:40

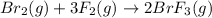
![\Delta H=\sum [n\times B.E(reactant)]-\sum [n\times B.E(product)]](/tpl/images/0714/8206/42942.png)
![\Delta H=[(n_{Br_2}\times B.E_{Br_2})+(n_{F_2}\times B.E_{F_2}) ]-[(n_{BrF_3}\times B.E_{BrF_3})]](/tpl/images/0714/8206/3f797.png)
![\Delta H=[(n_{Br_2}\times B.E_{Br-Br})+(n_{F_2}\times B.E_{F_F}) ]-[(n_{BrF_3}\times 3\times B.E_{Br-F})]](/tpl/images/0714/8206/a662a.png)
![\Delta H=[(1\times 193)+(3\times 155)]-[(2\times 3\times B.E_{Br-F})]](/tpl/images/0714/8206/a838d.png)