
Chemistry, 28.07.2020 23:01 alexis9658
According to the phase diagram for H20 what happens to the phases of water at 1 atm pressure as the temperature is increased from -10 degrees Celsius to 110 degrees
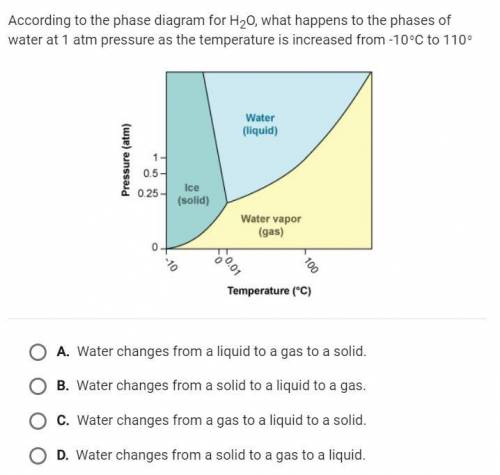

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1


Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
According to the phase diagram for H20 what happens to the phases of water at 1 atm pressure as the...
Questions




Chemistry, 28.09.2019 16:30



Mathematics, 28.09.2019 16:30





Mathematics, 28.09.2019 16:30




Mathematics, 28.09.2019 16:30

Health, 28.09.2019 16:30



Mathematics, 28.09.2019 16:30



