
Chemistry, 29.07.2020 21:01 hubbabubba0715
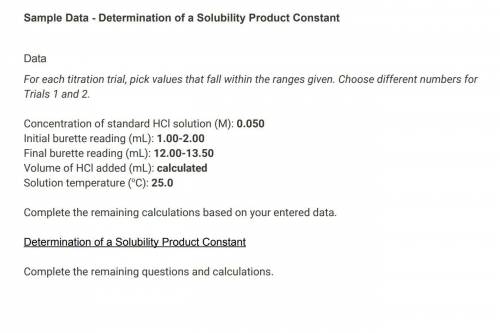
Concentration of standard HCl solution (M) 0.050 Saved Table view List view Trial 1 Trial 2 Initial burette reading (mL) 1.00 2.00 Final burette reading (mL) 12.00 13.50 Volume of HCl added (mL) 13.00 15.50 Solution temperature (°C) 25.0 25.0 (1pts) Average volume HCl added (mL) 14.25 Saved (2pts) Concentration of OH− (M) (2pts) Concentration of Ca2+ (M) (2pts) Value of Ksp for Ca(OH)2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
You know the right answer?
Concentration of standard HCl solution (M) 0.050 Saved Table view List view Trial 1 Trial 2 Initial...
Questions


Mathematics, 05.05.2020 13:40



Mathematics, 05.05.2020 13:40

Mathematics, 05.05.2020 13:40

Mathematics, 05.05.2020 13:40

Mathematics, 05.05.2020 13:40

Biology, 05.05.2020 13:40

History, 05.05.2020 13:40

English, 05.05.2020 13:40







Biology, 05.05.2020 13:40

History, 05.05.2020 13:40

Mathematics, 05.05.2020 13:40



