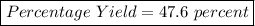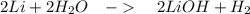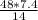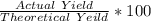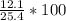Chemistry, 29.07.2020 09:01 20sgonzalez
What is the percent yield of lithium hydroxide from a reaction of 7.40g of lithium with 10.2g of water? The actual yield was measured to be 12.1g.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Perform the following mathematical operations and report the answer to the appropriate number of significant figures 5.87998 + 3.100
Answers: 2


Chemistry, 23.06.2019 01:10
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3

You know the right answer?
What is the percent yield of lithium hydroxide from a reaction of 7.40g of lithium with 10.2g of wat...
Questions




Mathematics, 19.05.2021 01:00

Mathematics, 19.05.2021 01:00


Health, 19.05.2021 01:00


Mathematics, 19.05.2021 01:00

Mathematics, 19.05.2021 01:00



Mathematics, 19.05.2021 01:00


Computers and Technology, 19.05.2021 01:00



Mathematics, 19.05.2021 01:00


History, 19.05.2021 01:00