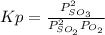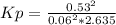Chemistry, 28.07.2020 20:01 arnold2619
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. An industrial chemist studying this reaction fills a 1.5 L flask with 0.59 atm of sulfur dioxide gas and 2.9 atm of oxygen gas at 35.0 °C. He then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of sulfur trioxide gas to be 0.53 atm.
Calculate the pressure equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. Round your answer to 2 significant digits.
Kp=.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1

Chemistry, 23.06.2019 09:00
What properties would have caused early researchers to name hydrogen "inflammable air”
Answers: 3

Chemistry, 23.06.2019 10:20
Based on the equation, how many grams of br2 are required to react completely with 29.2 grams of alcl3? alcl3 + br2 → albr3 + cl2 48.7 grams 52.6 grams 56.7 grams 61.3 grams
Answers: 3
You know the right answer?
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid...
Questions


Physics, 04.07.2019 19:30





Biology, 04.07.2019 19:30

Mathematics, 04.07.2019 19:30



History, 04.07.2019 19:30

Social Studies, 04.07.2019 19:30


History, 04.07.2019 19:30



English, 04.07.2019 19:30

Mathematics, 04.07.2019 19:30


Computers and Technology, 04.07.2019 19:30