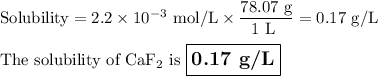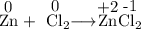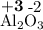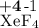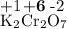Chemistry, 27.07.2020 23:01 yasyyas646646
Calculate the solubility of CaF2 in g/L (Ksp = 4.0 x 10-8). 2. What is the pH of a solution containing a hydrogen ion concentration of 3.0x10-4M? 3. What is the oxidizing agent and reducing agent in the following reaction: Zn(s) + Cl2(g) → ZnCl2(s) 4. Assign the oxidation numbers of the atoms in the following compounds. (a) Al2O3 (b) XeF4 (c) K2Cr2O7

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1

Chemistry, 23.06.2019 11:30
If this sedimentary rock layer is truly the oldest one of marine origin, what do you think that tells usabout the formation of earth's oceans?
Answers: 2
You know the right answer?
Calculate the solubility of CaF2 in g/L (Ksp = 4.0 x 10-8). 2. What is the pH of a solution containi...
Questions

English, 14.01.2021 19:20

Mathematics, 14.01.2021 19:20




Biology, 14.01.2021 19:20

Physics, 14.01.2021 19:20

Arts, 14.01.2021 19:20

Mathematics, 14.01.2021 19:20




Mathematics, 14.01.2021 19:20


Social Studies, 14.01.2021 19:20

Physics, 14.01.2021 19:20


Mathematics, 14.01.2021 19:20


Mathematics, 14.01.2021 19:20

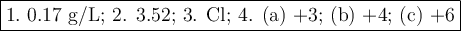
![K_{\text{sp }} = \text{[Ca$^{2+}$]}\text{[F$^{-}$]}^{2}= 4.0 \times 10^{-8}\\s(2s)^{2}=4.0 \times 10^{-8}\\4s^{3} = 4.0 \times 10^{-8}\\s^{3} = 1.0 \times 10^{-8}\\s =2.2 \times 10^{-3}\text{ mol/L}](/tpl/images/0713/9225/e2a2c.png)