

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1

Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3
You know the right answer?
The equilibrium constants for the chemical reaction N 2(g) + O 2(g) 2NO(g) are K P = 1.1 × 10 –3 and...
Questions




Mathematics, 19.10.2021 01:00


Computers and Technology, 19.10.2021 01:00






History, 19.10.2021 01:00


SAT, 19.10.2021 01:00

Business, 19.10.2021 01:00





Social Studies, 19.10.2021 01:00

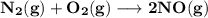
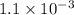
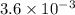
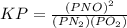
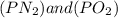 is defined in the denominator section and the value of
is defined in the denominator section and the value of 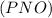 is defined in the numerator section that defines the value of KP is increases.so, the temperature of the KP will be KP ∝ (PNO).
is defined in the numerator section that defines the value of KP is increases.so, the temperature of the KP will be KP ∝ (PNO).

