
Chemistry, 26.07.2020 14:01 sarahhN7534
0.60 atm of SO3 and 0.30 atm of SO2are placed in a container and the system is allowed to reach equilibrium. Calculate the pressure of O2(g) at equilibrium.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
You know the right answer?
0.60 atm of SO3 and 0.30 atm of SO2are placed in a container and the system is allowed to reach equi...
Questions

Mathematics, 24.02.2021 18:50

Biology, 24.02.2021 18:50


English, 24.02.2021 18:50

Mathematics, 24.02.2021 18:50

Mathematics, 24.02.2021 18:50

Mathematics, 24.02.2021 18:50

Social Studies, 24.02.2021 18:50

Mathematics, 24.02.2021 18:50


Mathematics, 24.02.2021 18:50

Biology, 24.02.2021 18:50

Mathematics, 24.02.2021 18:50


Mathematics, 24.02.2021 18:50

Mathematics, 24.02.2021 18:50

Mathematics, 24.02.2021 18:50

Social Studies, 24.02.2021 18:50

Mathematics, 24.02.2021 18:50

Geography, 24.02.2021 18:50

![[O_2] = 4.8 *10^{-5} \ atm](/tpl/images/0713/4227/83355.png)
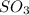 is
is ![[SO_3 ] = 0.63 \ atm](/tpl/images/0713/4227/96504.png)
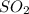 is
is ![[SO_ 2] = 0.30 \ atm](/tpl/images/0713/4227/bdacf.png)
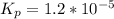
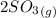 ⇔
⇔ 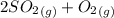
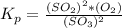
![[O_2] = \frac{k_p * [SO_3] ^2 }{[SO_2]^2}](/tpl/images/0713/4227/a8504.png)
![[O_2] = \frac{1.2 *10^{-5} * 0.60 ^2 }{0.30^2}](/tpl/images/0713/4227/296ec.png)



