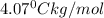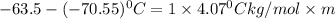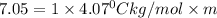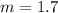Chemistry, 25.07.2020 20:01 maskoffvon
The freezing point of pure chloroform is -63.5°C, and its freezing point depression constant is 4.07°C•kg/mol. If the freezing point of a solution of benzoic acid in chloroform is -70.55°C, what is the molality of this solution? 0.58 m 1.7 m 16 m 17 m

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3


Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
The freezing point of pure chloroform is -63.5°C, and its freezing point depression constant is 4.07...
Questions



Mathematics, 21.02.2021 08:40

Mathematics, 21.02.2021 08:40

Biology, 21.02.2021 08:40


Advanced Placement (AP), 21.02.2021 08:40

Mathematics, 21.02.2021 08:40

Mathematics, 21.02.2021 08:40


Biology, 21.02.2021 08:40

Social Studies, 21.02.2021 08:40

History, 21.02.2021 08:50



SAT, 21.02.2021 08:50

Biology, 21.02.2021 08:50

Mathematics, 21.02.2021 08:50

Biology, 21.02.2021 08:50

Chemistry, 21.02.2021 08:50

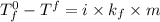
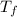 = freezing point of solution =
= freezing point of solution = 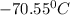
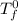 = freezing point of pure chloroform =
= freezing point of pure chloroform = 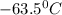
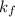 = freezing point constant of benzene =
= freezing point constant of benzene =