
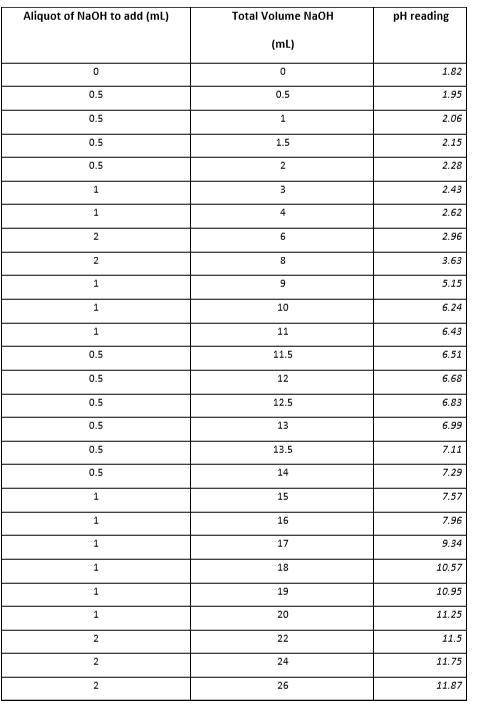
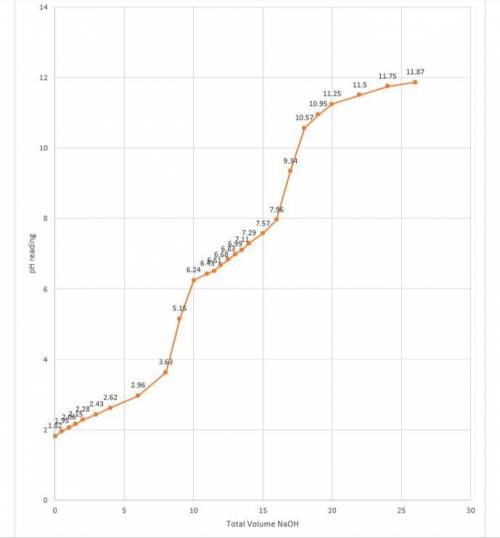
1.How could you determine the pH range over which a particular buffer system works best? 2.What are the pKa values for the various species of phosphoric acid and associated ions? Do your results agree with these values? Explain. 3.Provide an explanation for the shape of your graph for the Weak Acid/Strong Base titration. 4.Label the equivalence point/s, half equivalence points (corresponding to the pKas of phosphoric acid) and any buffer zones on your graph.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
1.How could you determine the pH range over which a particular buffer system works best? 2.What are...
Questions





Social Studies, 10.07.2019 01:30




Computers and Technology, 10.07.2019 01:30


Biology, 10.07.2019 01:30


Chemistry, 10.07.2019 01:30






Computers and Technology, 10.07.2019 01:30

Computers and Technology, 10.07.2019 01:30



