

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
You know the right answer?
The equilibrium constant for the reaction is 1.1 x 106 M. HONO(aq) + CN-(aq) ⇋ HCN(aq) + ONO-(aq) Th...
Questions



Mathematics, 18.03.2022 16:10

Mathematics, 18.03.2022 16:10




Computers and Technology, 18.03.2022 16:10

Mathematics, 18.03.2022 16:10



Spanish, 18.03.2022 16:10





Mathematics, 18.03.2022 16:10



 M.
M.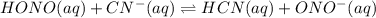
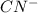 is a stronger base than
is a stronger base than 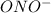
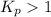 ; the reaction is product favoured.
; the reaction is product favoured.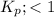 ; the reaction is reactant favored.
; the reaction is reactant favored.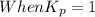 ; the reaction is in equilibrium.
; the reaction is in equilibrium.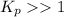 , the reaction will be product favoured and as it is a acid base reaction where
, the reaction will be product favoured and as it is a acid base reaction where 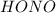 acts as acid by donating
acts as acid by donating 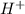 ions and
ions and 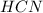 conjugate acid.
conjugate acid.

