
Chemistry, 25.07.2020 03:01 jessica112776
Use the balanced combustion reaction above to calculate the enthalpy of combustion for C8H16. C8H16(1)= -174.5kJ/mol. I have no clue how to start this question and need help including the formulas so I know how to do it and some step by step commentary.


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1


Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
Use the balanced combustion reaction above to calculate the enthalpy of combustion for C8H16. C8H16(...
Questions

Mathematics, 06.11.2020 22:10

Mathematics, 06.11.2020 22:10


Mathematics, 06.11.2020 22:10

History, 06.11.2020 22:10





Mathematics, 06.11.2020 22:20

Mathematics, 06.11.2020 22:20

Mathematics, 06.11.2020 22:20

Mathematics, 06.11.2020 22:20


Mathematics, 06.11.2020 22:20

Mathematics, 06.11.2020 22:20


English, 06.11.2020 22:20

History, 06.11.2020 22:20

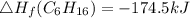
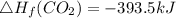
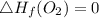
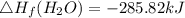
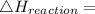 8 x - 393.5 - 8 x 285.82 + 174.5x 1
8 x - 393.5 - 8 x 285.82 + 174.5x 1 

