

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
How many moles of ZnCl2 will be produced from 61.0 g of Zn, assuming HCl is excess?...
Questions

Physics, 03.07.2020 21:01




Mathematics, 03.07.2020 21:01




Mathematics, 03.07.2020 21:01

English, 03.07.2020 21:01


Geography, 03.07.2020 21:01

Mathematics, 03.07.2020 21:01





Physics, 03.07.2020 21:01

Mathematics, 03.07.2020 21:01

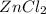 will be produced.
will be produced.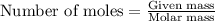
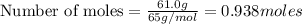
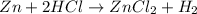
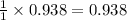 moles of
moles of 

