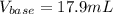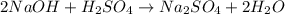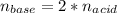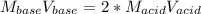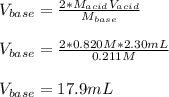Chemistry, 23.07.2020 21:01 shongmadi77
Calculate the volume, in milliliters, of a 0.211 M solution of NaOH that will completely neutralize each of the following. 2.30 mL of a 0.820 M solution of H2SO4. Express the volume in milliliters to three significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1


Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
Calculate the volume, in milliliters, of a 0.211 M solution of NaOH that will completely neutralize...
Questions



Mathematics, 17.08.2019 05:10

History, 17.08.2019 05:10







English, 17.08.2019 05:10






History, 17.08.2019 05:10

Mathematics, 17.08.2019 05:10