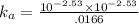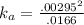Chemistry, 23.07.2020 20:01 brendancrow5927
Enough of a monoprotic weak acid is dissolved in water to produce a 0.01660.0166 M solution. The pH of the resulting solution is 2.532.53 . Calculate the Ka for the acid.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1


Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1

Chemistry, 23.06.2019 17:40
When 7.00g of hydrogen react with 70.0g of nitrogen, hydrogen is considered the limiting reactant because
Answers: 1
You know the right answer?
Enough of a monoprotic weak acid is dissolved in water to produce a 0.01660.0166 M solution. The pH...
Questions




History, 06.07.2019 16:10

Biology, 06.07.2019 16:10


Geography, 06.07.2019 16:10


Mathematics, 06.07.2019 16:10





Geography, 06.07.2019 16:10

Health, 06.07.2019 16:10

History, 06.07.2019 16:10



Biology, 06.07.2019 16:10

Mathematics, 06.07.2019 16:10

![[ H^+]=10^{-2.53}](/tpl/images/0711/9511/3a08a.png)
![[ X^-]=10^{-2.53}](/tpl/images/0711/9511/d1b85.png)