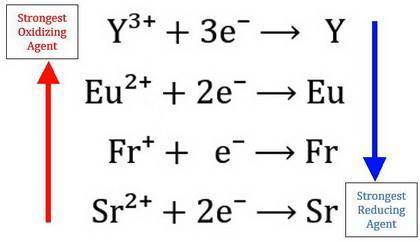Chemistry, 23.07.2020 09:01 Conpolice1309
. The four metals, Strontium(Sr), Francium (Fr), Yttrium (Y), and Europium (Eu), in separate experiments, are dipped in aqueous solutions of SrNO3, FrNO3, Y(NO3)3, and Eu(NO3)2. The following results are obtained: 1. Yttrium metal does not react with any of the solutions 2. Strontium metal reacts with all of the other metals solutions 3. Francium metal reacts in a solution of Eu(NO3)2 a) List the four oxidizing agents in order from strongest to weakest by creating a small reduction table. Explain your reasoning below b) List the four reducing agents in order from strongest to weakest

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
. The four metals, Strontium(Sr), Francium (Fr), Yttrium (Y), and Europium (Eu), in separate experim...
Questions




Health, 12.12.2021 09:50


English, 12.12.2021 09:50

English, 12.12.2021 09:50

Mathematics, 12.12.2021 09:50

English, 12.12.2021 09:50

Business, 12.12.2021 09:50

English, 12.12.2021 09:50

Mathematics, 12.12.2021 09:50

Mathematics, 12.12.2021 09:50

Advanced Placement (AP), 12.12.2021 09:50


Mathematics, 12.12.2021 09:50

Social Studies, 12.12.2021 09:50

Social Studies, 12.12.2021 09:50


Advanced Placement (AP), 12.12.2021 09:50