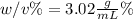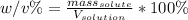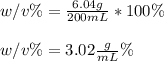An aqueous solution of cobalt(II) fluoride, , is made by dissolving 6.04 grams of cobalt(II) fluoride in sufficient water in a 200. mL volumetric flask, and then adding enough water to fill the flask to the mark. What is the weight/volume percentage of cobalt(II) fluoride in the solution

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
You know the right answer?
An aqueous solution of cobalt(II) fluoride, , is made by dissolving 6.04 grams of cobalt(II) fluorid...
Questions


English, 04.04.2020 20:16

History, 04.04.2020 20:16

Mathematics, 04.04.2020 20:16

Mathematics, 04.04.2020 20:16



Mathematics, 04.04.2020 20:16

History, 04.04.2020 20:16


Biology, 04.04.2020 20:17

Mathematics, 04.04.2020 20:17

History, 04.04.2020 20:17

Business, 04.04.2020 20:17

Mathematics, 04.04.2020 20:17

Mathematics, 04.04.2020 20:17