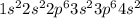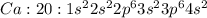Chemistry, 22.07.2020 20:01 yselahernandez02
where 1s, 2s, and 2p are the occupied subshells, and the superscript "2" is the number of electrons in each of these subshells. Use the rules for determining electron configurations to write the electron configuration for Ca. Express your answer in complete form in order of orbital filling. For example, 1s22s2 should be entered as 1s^22s^2.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1


Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
where 1s, 2s, and 2p are the occupied subshells, and the superscript "2" is the number of electrons...
Questions



Geography, 21.10.2020 16:01

Mathematics, 21.10.2020 16:01





English, 21.10.2020 16:01

Arts, 21.10.2020 16:01





Chemistry, 21.10.2020 16:01



Physics, 21.10.2020 16:01

English, 21.10.2020 16:01