
Chemistry, 22.07.2020 06:01 faithtunison
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. An industrial chemist studying this reaction fills a
75.0 L tank with 3.8 mol of sulfur dioxide gas and 7.0 mol of oxygen gas, and when the mixture has come to equilibrium measures the amount of sulfur trioxide
gas to be 1.5 mol
Calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. Round your answer to 2
significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1


Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

You know the right answer?
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid...
Questions


Social Studies, 19.11.2020 09:10

Mathematics, 19.11.2020 09:10



Mathematics, 19.11.2020 09:10


English, 19.11.2020 09:10







Social Studies, 19.11.2020 09:10

Mathematics, 19.11.2020 09:10


SAT, 19.11.2020 09:10

Mathematics, 19.11.2020 09:10

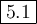
![K_{\text{eq}} = \dfrac{\text{[SO$_{3}$]}^{2}}{\text{[SO}_{2}]^{2}\text{[O$_{2}$]}} = \dfrac{0.0200^{2}}{0.0307^{2}\times0.0833} =\mathbf{5.1}\\\\\text{The value of the equilibrium constant is $\large \boxed{\mathbf{5.1}}$}](/tpl/images/0711/0231/1d368.png)


