
In a study of the decomposition of the compound XX via the reaction
X(g)⇌Y(g)+Z(g)X(g)⇌Y(g)+Z(g)
the following concentration-time data were collected:
Time (min)(min) [X](M)[X](M)
0 0.467
1 0.267
2 0.187
3 0.144
4 0.117
5 0.099
6 0.085
7 0.075
Given that the rate constant for the decomposition of hypothetical compound XX is is 1.60 M^−1⋅min^−1. Calculate the concentration of XX after 18.0 minmin .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1


Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1
You know the right answer?
In a study of the decomposition of the compound XX via the reaction
X(g)⇌Y(g)+Z(g)X(g)⇌Y(g)+Z(g)
Questions

Geography, 18.03.2022 14:00











English, 18.03.2022 14:00



Computers and Technology, 18.03.2022 14:00

English, 18.03.2022 14:00




Mathematics, 18.03.2022 14:00

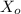 ]"
]"

