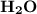Indicate the types of forces that are involved between the solute and solvent when forming a homogeneous solution between CH3CH2CH2CH2CH3 and CH3CH2CH2CH2CH2CH3.
a. dispersion forces
b. dipole-dipole forces
c. hydrogen bonding
d. ion-dipole forces
2. Indicate the types of forces that are involved between the solute and solvent when forming a homogeneous solution between LiNO3 and H2O.
a. dispersion forces
b. dipole-dipole forces
c. hydrogen bonding
d. ion-dipole forces

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
Indicate the types of forces that are involved between the solute and solvent when forming a homogen...
Questions

History, 01.04.2020 01:15

Mathematics, 01.04.2020 01:15


English, 01.04.2020 01:15




English, 01.04.2020 01:15


Mathematics, 01.04.2020 01:16

Computers and Technology, 01.04.2020 01:16

Mathematics, 01.04.2020 01:16

Physics, 01.04.2020 01:16


Mathematics, 01.04.2020 01:16

Mathematics, 01.04.2020 01:16


Mathematics, 01.04.2020 01:16

Mathematics, 01.04.2020 01:16

Biology, 01.04.2020 01:16

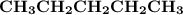 and
and 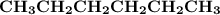
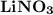 and
and