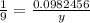Chemistry, 20.07.2020 06:01 jjjones2233
Consider the combustion of octane:
2C8H18(g) + 2502(g) ===> 16CO2(g) + 18H2O(g)
Calculate the mass of water (H20) produced from 11.2 grams of octane (C3H18) assuming excess
oxygen.
15.9 g
O 0.196 g
O 4.31 g
1.77g
14.1g

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1
You know the right answer?
Consider the combustion of octane:
2C8H18(g) + 2502(g) ===> 16CO2(g) + 18H2O(g)
Calculate...
Calculate...
Questions

Mathematics, 13.11.2020 18:50



History, 13.11.2020 18:50




Mathematics, 13.11.2020 18:50

History, 13.11.2020 18:50

Arts, 13.11.2020 18:50

SAT, 13.11.2020 18:50

Mathematics, 13.11.2020 18:50





Mathematics, 13.11.2020 18:50


Physics, 13.11.2020 18:50