Chemistry, 19.07.2020 17:01 Aurionna101
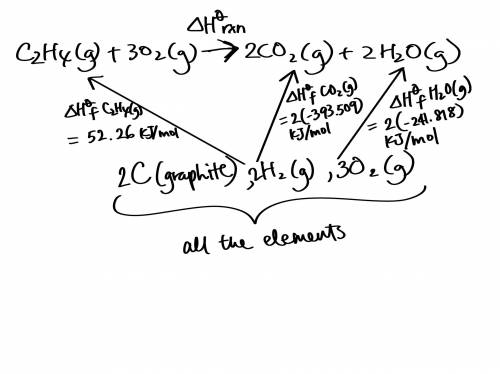
Use the standard enthalpies of formation for the reactants and products to solve for the ΔHrxn for the following reaction. (The ΔHf of C2H4 is 52.26 kJ/mol, CO2 is -393.509 kJ/mol, and H2O is -241.818 kJ.)
C2H4 (g) + 3O2(g) 2CO2 (g) + 2H2O(g)
ΔHrxn = (-345.64 kJ, -583.07 kJ, or -1,322.91 kJ).
The reaction is: (Endothermic or Exothermic).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
Use the standard enthalpies of formation for the reactants and products to solve for the ΔHrxn for t...
Questions


Social Studies, 17.03.2020 02:05

Mathematics, 17.03.2020 02:05

Mathematics, 17.03.2020 02:05



Mathematics, 17.03.2020 02:06

Mathematics, 17.03.2020 02:06

Mathematics, 17.03.2020 02:06

Mathematics, 17.03.2020 02:06


Mathematics, 17.03.2020 02:06





Computers and Technology, 17.03.2020 02:06


History, 17.03.2020 02:06