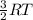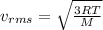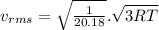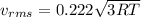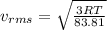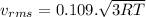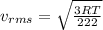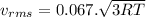Chemistry, 18.07.2020 04:01 Knownothing
A flask contains a mixture of neon Ne, krypton Kr, and radon Rn gases. (Hint: The molar mass of the is Ne 20.180 g/mol, of the Kr is 83.80g/mol, and of the Kr 222g/mol)
(A) Compare the average kinetic energies of the Ne and Kr.
(B) Comparethe average kinetic energies of the Kr and Rn.
(C) Compare the average kinetic energies of the Rn and Ne.
(D) Compare the root-mean-square speeds of the Ne and Kr.
(E) Compare the root-mean-square speeds of the Kr and Rn.
(F) Compare the root-mean-square speeds of the Rn and Ne.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 22.06.2019 23:30
Why do oxygen have a strong attractive force for electrons
Answers: 2
You know the right answer?
A flask contains a mixture of neon Ne, krypton Kr, and radon Rn gases. (Hint: The molar mass of the...
Questions


History, 28.07.2019 21:10


Business, 28.07.2019 21:10


History, 28.07.2019 21:10


Biology, 28.07.2019 21:10

Business, 28.07.2019 21:10


Business, 28.07.2019 21:10

Chemistry, 28.07.2019 21:10




Mathematics, 28.07.2019 21:10

Mathematics, 28.07.2019 21:10