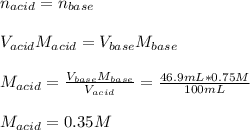Chemistry, 17.07.2020 20:01 mrashrafkotkaat
The reaction of perchloric acid (HClO4) with lithium hydroxide (LiOH) is described by the equation: HClO4 + LiOH → LiClO4 + H2O Suppose 100 mL of perchloric acid is neutralized by exactly 46.9 mL of 0.75 M lithium hydroxide. What is the concentration of the perchloric acid? A. 0.35 M B. 0.47 M C. 0.63 M D. 1.60 M

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1
You know the right answer?
The reaction of perchloric acid (HClO4) with lithium hydroxide (LiOH) is described by the equation:...
Questions



Mathematics, 13.12.2021 21:40


Mathematics, 13.12.2021 21:40

History, 13.12.2021 21:40

English, 13.12.2021 21:40

English, 13.12.2021 21:40

Medicine, 13.12.2021 21:40

English, 13.12.2021 21:40

History, 13.12.2021 21:40







Mathematics, 13.12.2021 21:40

History, 13.12.2021 21:40

Mathematics, 13.12.2021 21:40