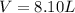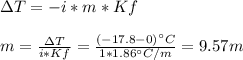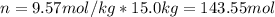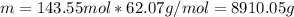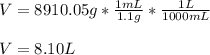Chemistry, 17.07.2020 21:01 stacysadousky
How many liters of ethylene glycol antifreeze (C 2H 6O 2) would you add to your car radiator containing 15.0 L of water if you needed to protect your engine to –17.8°C? (The density of ethylene glycol is 1.1 g/mL. For water, K f = 1.86°C/m.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 10:00
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
How many liters of ethylene glycol antifreeze (C 2H 6O 2) would you add to your car radiator contain...
Questions

Physics, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00

Physics, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00


Mathematics, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00




Biology, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00

English, 05.02.2021 01:00


Mathematics, 05.02.2021 01:00

History, 05.02.2021 01:00

Spanish, 05.02.2021 01:00

Mathematics, 05.02.2021 01:00

Chemistry, 05.02.2021 01:00