
Chemistry, 16.07.2020 17:01 payshencec21
The equilibrium constant, Kc, for the following reaction is 55.6 at 698 K:
H2(g) + I2(g) 2HI(g)
Calculate the equilibrium concentrations of reactants and product when 0.234 moles of H2 and 0.234 moles of I2 are introduced into a 1.00 L vessel at 698 K.
[H2] = M
[I2] = M
[HI] = M

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1

Chemistry, 23.06.2019 04:20
Calculate the mass of 0.750 mol of the following substance. na3po4.
Answers: 1
You know the right answer?
The equilibrium constant, Kc, for the following reaction is 55.6 at 698 K:
H2(g) + I2(g) 2HI(g)
Questions


Mathematics, 02.02.2020 22:52


History, 02.02.2020 22:52

Biology, 02.02.2020 22:52





Mathematics, 02.02.2020 22:53

Mathematics, 02.02.2020 22:53


History, 02.02.2020 22:53

Mathematics, 02.02.2020 22:53



Biology, 02.02.2020 22:53

English, 02.02.2020 22:53

Mathematics, 02.02.2020 22:53

![[I_2]=[H_2]=0.369M](/tpl/images/0707/8736/ed0a2.png)
![[HI]=0.0495M](/tpl/images/0707/8736/4fca5.png)
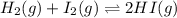
![Kc=\frac{[HI]^2}{[I_2][H_2]}](/tpl/images/0707/8736/bf8a4.png)
 (considering the ICE procedure) is written as:
(considering the ICE procedure) is written as:![55.6=\frac{(2x)^2}{([I_2]_0-x)([H_2]_0-x)}](/tpl/images/0707/8736/e5efd.png)
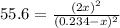
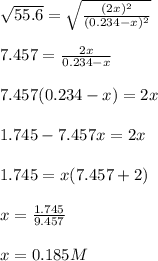
![[I_2]=[H_2]=2*0.185M=0.369M](/tpl/images/0707/8736/ec590.png)
![[HI]=0.234-0.185=0.0495M](/tpl/images/0707/8736/318f7.png)


