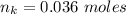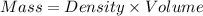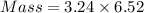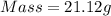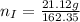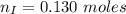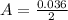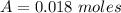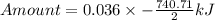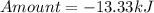Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
On a distance vs time graph the line of an object at rest is a
Answers: 1

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
How much heat is liberated at constant pressure when 1.41 g of potassium metal reacts with 6.52 mL o...
Questions

Mathematics, 05.12.2020 03:00


History, 05.12.2020 03:00




Mathematics, 05.12.2020 03:00


History, 05.12.2020 03:00



English, 05.12.2020 03:00

Mathematics, 05.12.2020 03:00


Mathematics, 05.12.2020 03:00





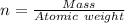
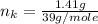 ---where 39 is the atomic weight of potassium
---where 39 is the atomic weight of potassium